26+ cell potential calculator
Quick Tips Calculating Standard Cell Potentials Loading. Web Calculating Standard Cell Potentials Read Chemistry CK-12 Foundation Calculating Standard Cell Potentials Introduces cell potentials and discusses how to mathematically predict reduction potential of different types of chemical cells.
Nernst Equation Calculator Calculate Equilibrium Potential
Calculate the overall cell potential of the following reaction.

. R Gas constant. Web The potential of the working electrode determines what kind of redox processes may occur at the electrodes surface. Web The cell potential of a cell is the potential difference occurring between the two electrodes of the cell and arises due to the transfer of electrons through the external circuit of a cell that has not reached equilibrium.
981 mathrm ms2 981 ms2 or. Web To calculate these standard cell potential for our zinc copper cell were going to use what are called Standard Reduction Potentials. Web This chemistry video tutorial explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell.
The data presented in this calculator concerns the temperature of 25 C. Mg Mg2 2e- where E₀ 238 V Pb2 2e- Pb where E₀ -013 V First we need to write down the total reaction and calculate the total standard redox potential. 2 1 e Ag aq Ag s E 0337 V.
Web Nernst Equation Calculator. Ec Eca Ea Where Ec is the cell potential Eca is the cathode potential Ea is the anode potential Cell Potential Definition Cell potential is defined as the difference between the cathode potential and the anode potential in a battery cell. 981 m s 2.
Web Cell Potential Calculations E cell ex. It explains how to calculate the cell potential of a concentration cel. Ecirc E circ_ cathode - E circ_ anode E.
Standard Reduction Potentials are symbolized by E naught of reduction. Web We will use the Nernst equation calculator to find the reduction potential of a cell basing on the following reactions. Web The following formula is used to calculate a cell potential.
Cus 2Fe 3 aq Cu 2 aq 2Fe 2 aq break up into half-reactions. Web The cell potential calculator provides a useful tool for scientists and engineers to estimate the potential difference between the cathode and anode of an electrochemical cell and to understand the electrochemical reactions that take place within the cell. T he net reaction of a voltaic cell constructed from a standard zinc electrode and a standard copper electrode is obtained by adding the two half-reactions together.
Below is our Nernst Equation Calculator. Pb2aq Mg s Mg2aq Pb s. T Temperature.
N The number of electrons transferred per cell reaction. Web The standard potential depends on the temperature. Web Here we need to calculate the cell potential for a zinc-copper cell where the concentration of zinc two plus ions and the concentration of copper two plus ions in solution is one molar and were at 25 degrees C.
E 0763 V. Web The easiest way to calculate gravitational potential energy is to use our potential energy calculator. Gravitational acceleration which on Earth amounts to.
This tool estimates the potential energy on the basis of three values. When describing a galvanic. O2g 4Haq 4Br-aq 2H2O l 2Br2l 1.
The cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. Web An online Nernst equation calculator can calculate the equilibrium potential for an ion based on its concentration and its charge. E o Standard Electrode potential of the cell.
Write the half-reactions for each process. Zn s Zn 2 aq 2e. The mass of the object.
E cell Electromotive force of the cell. Web Calculating the Cell Potential. Web Calculate the cell potential for the following reaction when the pressure of the oxygen gas is 250 atm the hydrogen ion concentration is 010 molL and the bromide ion concentration is 025 molL.
Found a content error. And these refer to the voltages for half reactions that are written as reduction half reactions. Web This chemistry video tutorial provides a basic introduction into concentration cells.
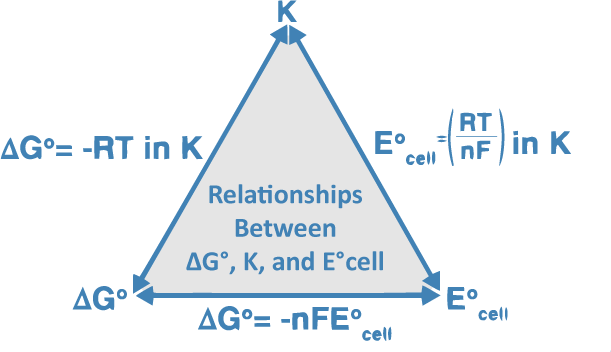
Knowing the potentials of half-cells cathode and anode we can calculate the potential of a cell composed of them. The standard cell potential for an electrochemical cell can be determined by finding the difference between the two half-cells reduction potentials. Extremely negative potentials will probably reduce analytes while positive potentials are likely to oxidize them.
E E c a t h o d e E a n o d e.

Chapter 18 Electrochemistry Ppt Download
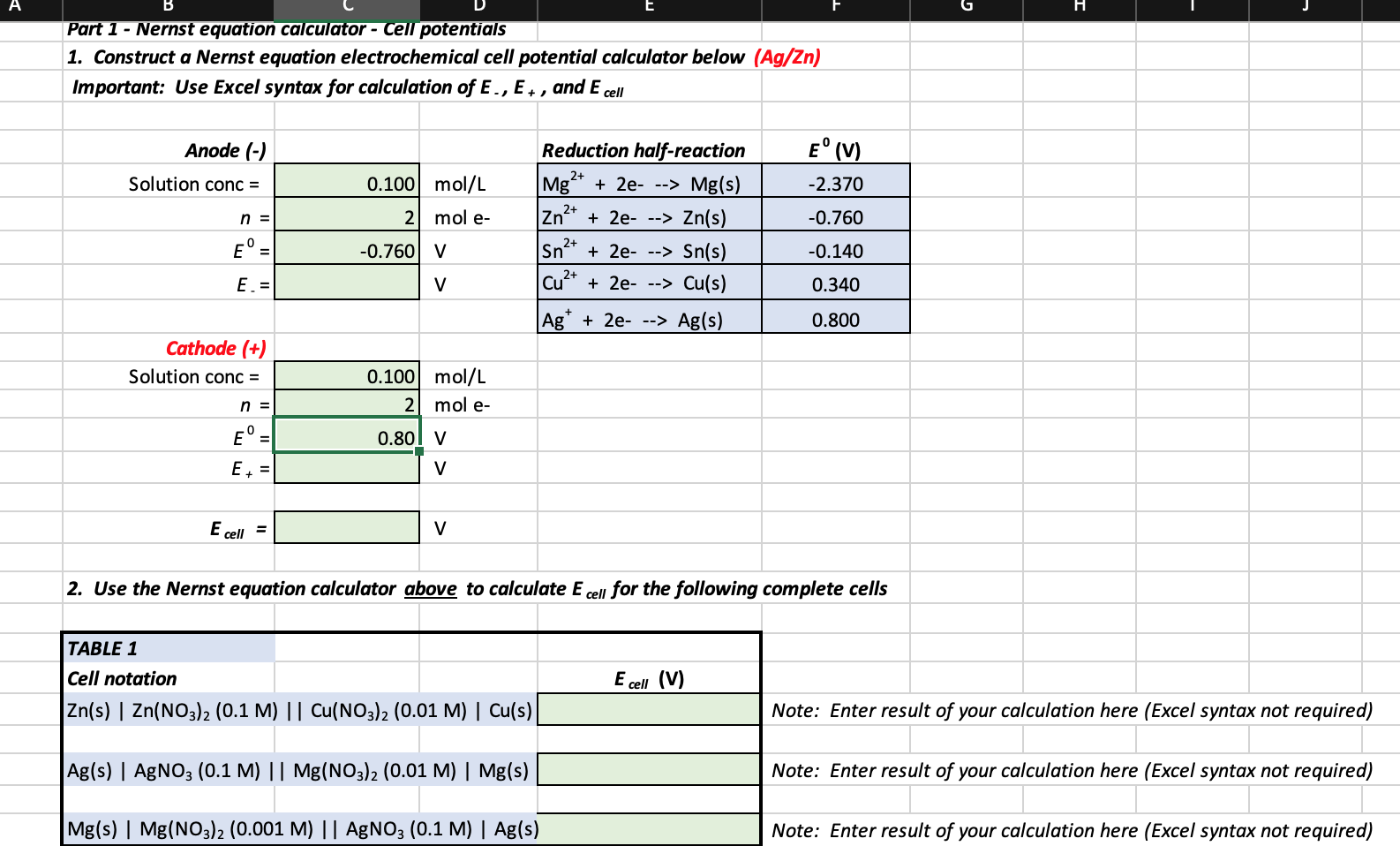
Solved A E H Part 1 Nernst Equation Calculator Cell Chegg Com

Isotopic Resolution Of Protein Complexes Up To 466 Kda Using Individual Ion Mass Spectrometry Analytical Chemistry
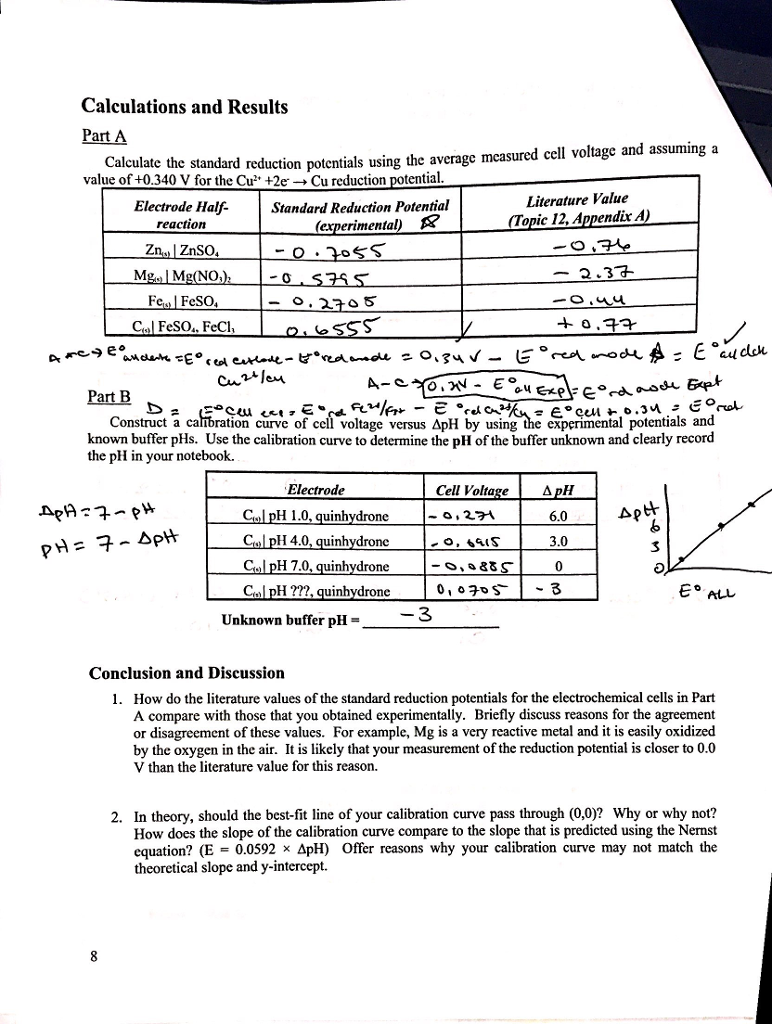
Solved Calculate The Standard Reduction Potentials Using The Chegg Com

Performance Of Statistical Methods For Meta Analysis When True Study Effects Are Non Normally Distributed A Simulation Study Evangelos Kontopantelis David Reeves 2012
Nernst Equation Calculator Calistry
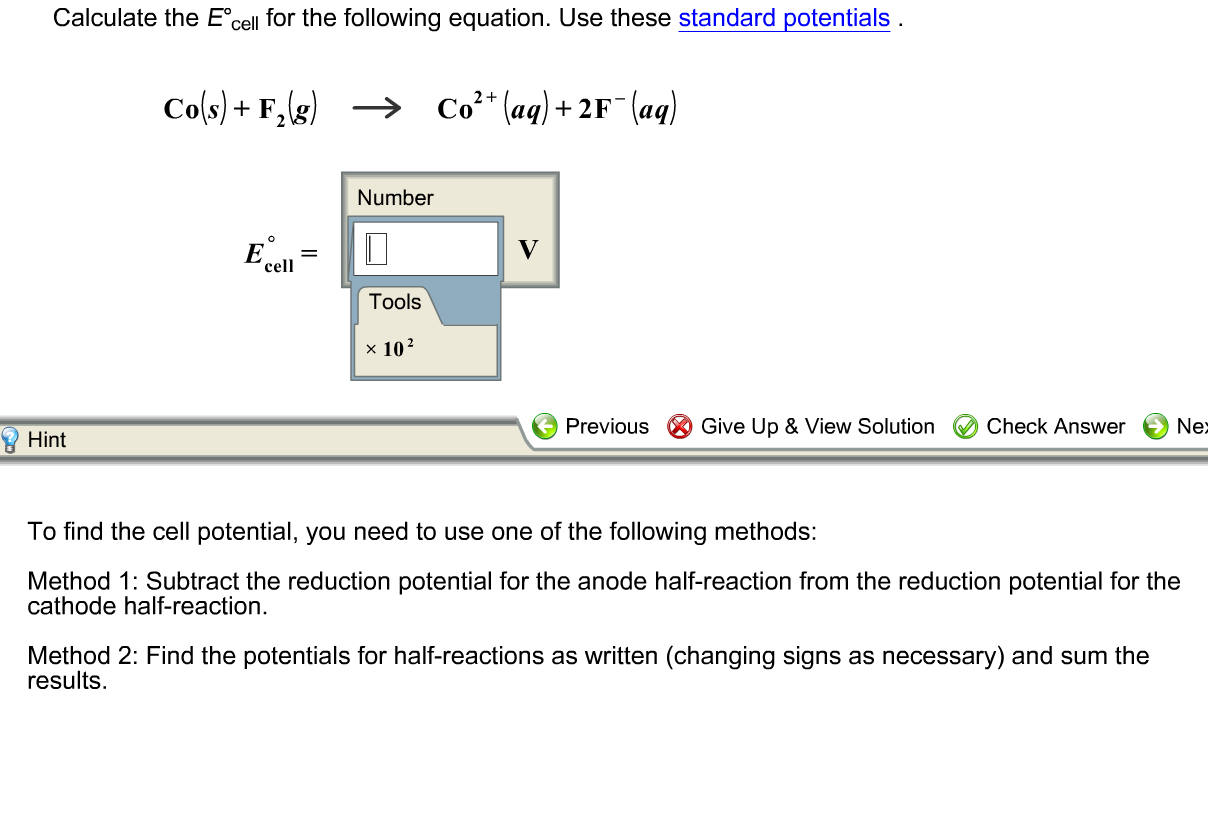
Solved Calculate The E Degree Cell For The Following Chegg Com

Vascular Deficiencies In Renal Organoids And Ex Vivo Kidney Organogenesis Sciencedirect
Calculator For Converting Potentials To Another Reference Electrode Gamry Instruments

Chapter 20 Electrochemistry Ppt Download
Release Of Nanodiscs From Charged Nano Droplets In The Electrospray Ionization Revealed By Molecular Dynamics Simulations Communications Chemistry

Nernst Equation An Overview Sciencedirect Topics
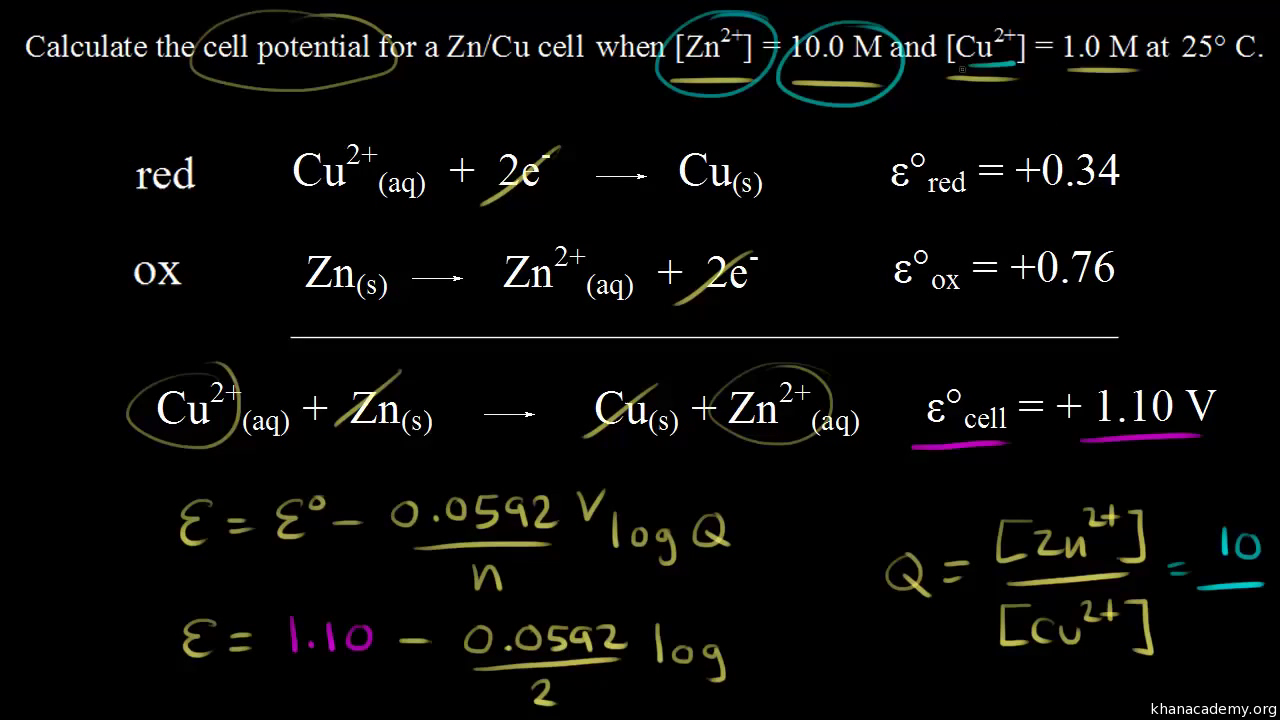
Using The Nernst Equation Video Khan Academy
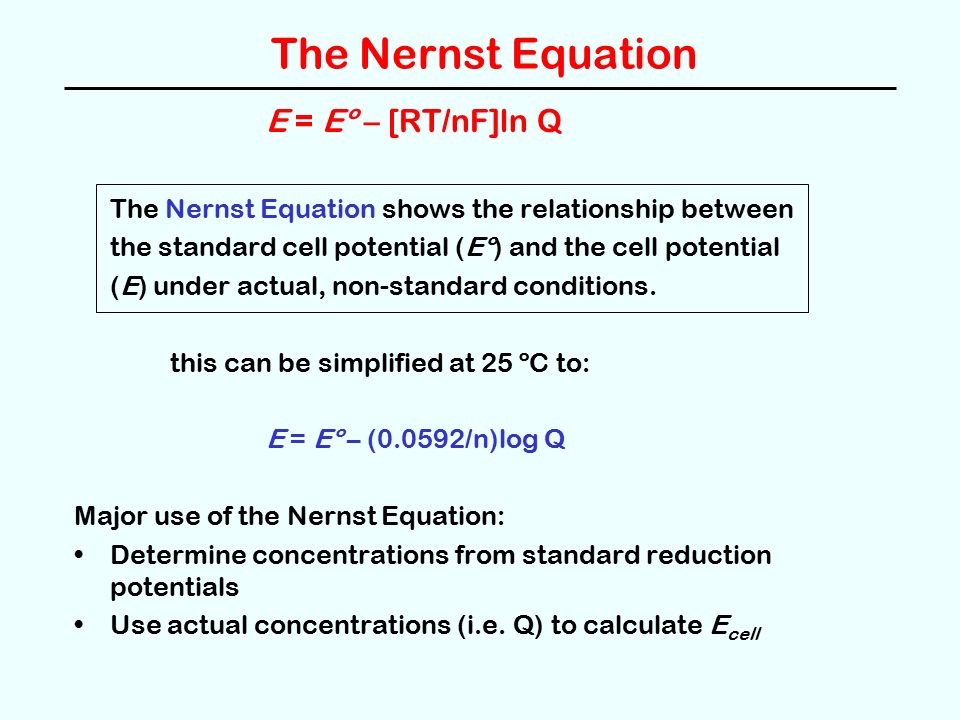
Oxidation Reduction Reactions Ppt Video Online Download

19 Electrochemistry Ppt Download
Nernst Equation Calculator Calistry

Nernst Equation An Overview Sciencedirect Topics